Incidence or T cell lymphoma in patients diagnosed with multiple myeloma or diffuse large B-cell lymphoma
A Komodo Real World Evidence analysis
Gurbakhash Kaur, M.D.1
Eloisa Riva, M.D.2
Saurabh Chhabra, M.D.3
Allison Rosenthal, D.O.3
Rafael Fonseca, M.D.3
1 UTSW Harold C. Simmons Comprehensive Cancer Center
2 Hospital del Clinicas Dr. Manuel Quintela, Montevideo, Uruguay
3 Mayo Clinic in Arizona, Phoenix, AZ
Corresponding author:
Rafael Fonseca, M.D.
Chief Innovation Officer
Getz Family Professor of Cancer
Distinguished Mayo Investigator
Mayo Clinic in Arizona
(480) 342-0440
Keywords: #myeloma, #mmsm, #CART, diffuse large B cell lymphoma, #DLBCL, #LymSM, T cell lymphoma, CART.
Abstract
The risk of T-cell malignancies following CAR-T therapy, though relatively rare, remains a serious concern. The Food and Drug Administration (FDA) recently issued a warning on the risk of T cell malignancy following BCMA or CD19 directed CAR-T therapy in multiple myeloma (MM) and diffuse large B cell lymphoma (DLBCL), citing 20 cases of T cell lymphoma (TCL) in patients who received CAR-T targeting BCMA or CD19.[1] Considering the potential oncogenicity of CAR-T therapy, and the impact of the FDA warning on a patient’s decision to receive an important lifesaving CAR-T cell therapy, it is imperative to investigate on the background incidence and prevalence of TCL in patients with MM or DLBCL. To address this, we conducted a real-world evidence (RWE) analysis from Komodo Health’s healthcare map to identify the prevalence and incidence of TCL in patients newly diagnosed with MM and DLBCL from 2018-2022 based on billing interactions for TCL.
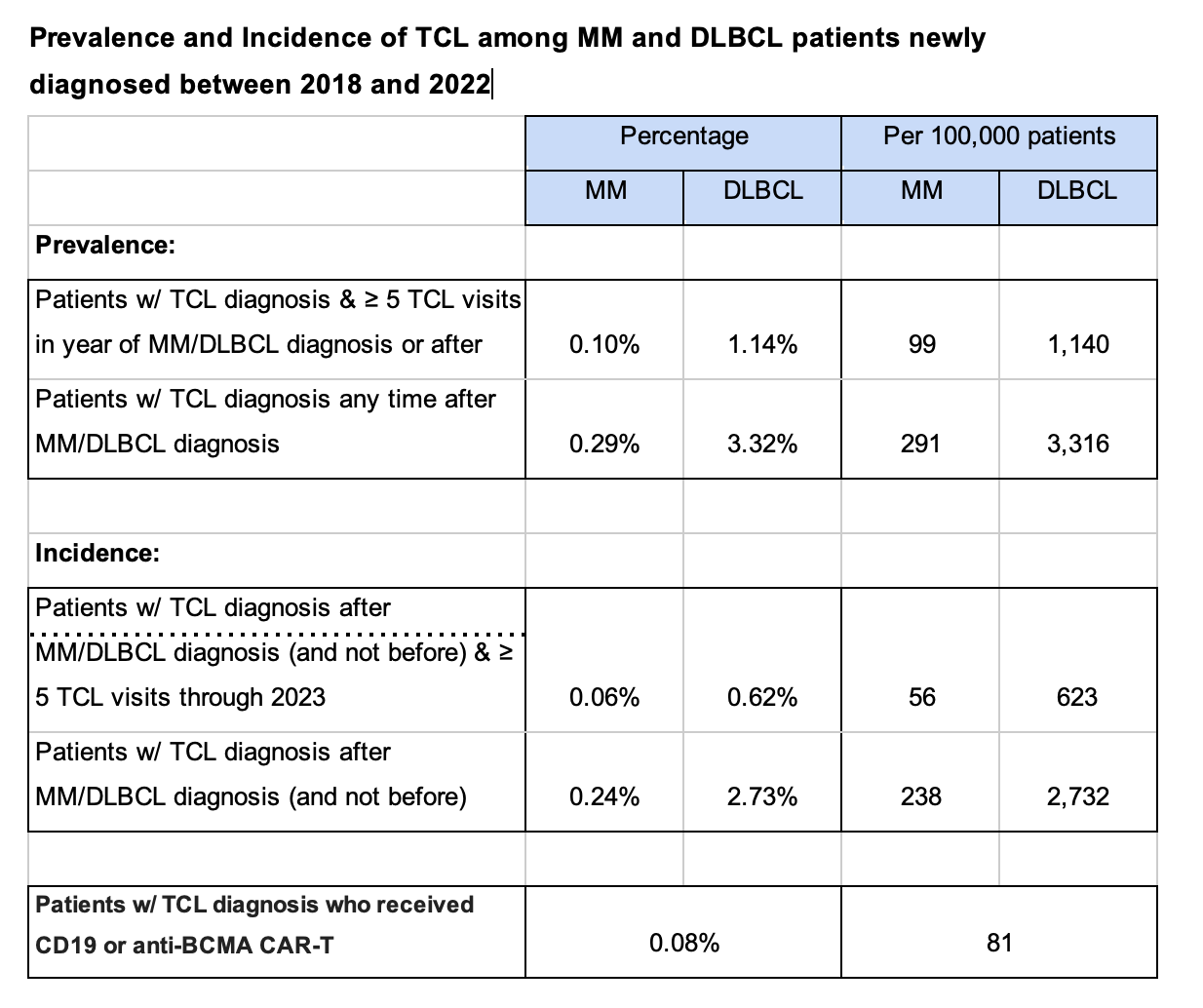
In MM, the prevalence of TCL was 0.10% (99 per 100,000 patients) with ≥ 5 billable TCL interactions, rising to 0.29% (291 per 100,000 MM patients) with ≥ 1 billable TCL interaction. Similarly, the incidence of TCL was 0.06% (56 cases per 100,000 patients) with ≥ 5 billable interactions and 0.24% (238 cases per 100,000 patients) with ≥ 1 billable interaction.
In DLBCL, the prevalence of TCL was 1.14% (1,140 per 100,000 patients) with ≥ 5 billable interactions and 3.32% (3,316 per 100,000 patients) with ≥ 1 billable interaction. The incidence of TCL in DLBCL was 0.62% (623 per 100,000 patients) and 2.73% (2,732 cases per 100,000 patients) with ≥ 5 and ≥1 billable interactions, respectively.
This compares to a rate of ~81 reported TCL cases per 100,000 patients in the FDA communication (22 cases in over 27,000 CAR-T cell therapies administered) treated with BCMA or CD19 CAR-T therapies. Our analysis highlights that the background incidence of TCL amongst patients with MM or DLBCL is comparable to that reported in patients who have received CAR-T therapy.
Introduction
On November 28, 2023, the FDA announced that they would be investigating the serious risk of T-cell malignancy following BCMA or CD19-directed CAR-T, for MM and DLBCL. The FDA indicated that they had identified 20 cases [1] of TCL in patients who received the aforementioned therapies from clinical trials or post marketing adverse event data sources. In a more recent and separate public communication the FDA reported 22 cases in over 27,000 CAR-T treated US patients (source: Peter Marks MD, ARM Cell & Gene State of the Industry Briefing, January 8, 2024, link).
To contextualize this risk, we sought to understand background rates of TCL in patients with MM and DLBCL. We realize that CD19 CAR-Ts will be used in other B cell neoplasias but chose to focus on the background rate of TCL for patients with DLBCL to simplify the analysis.
Materials and methods
We performed a RWE analysis based on data from Komodo Health’s healthcare map. Annual MM and DLBCL incidence rates were estimated by assessing the number of patients who were newly diagnosed with MM and DLBCL. We started by identifying newly diagnosed MM and DLBCL patients between 2018 and 2022 based on ICD10 diagnosis codes (see appendix) and receipt of relevant treatment within one year of the original diagnosis. For MM, we included patients who received at least one dose of bortezomib, lenalidomide, daratumumab, or carfilzomib. For DLBCL, we included patients who received at least one dose of rituximab, cyclophosphamide, or doxorubicin. Additionally, we required that all patients have at-least five billable MM or DLBCL interactions with a healthcare provider tagged with the relevant ICD10 code within a two year timeframe of diagnosis to minimize the number of miscoded patients (i.e., if the patient was diagnosed in April, 2018, we counted the number of billable interactions from Jan 1, 2018 to Dec 31, 2019 and only considered the patient to have a MM or DLBCL diagnosis if there were 5 or more billable interactions with a relevant ICD10 code).
We estimated both the prevalence and incidence of TCL in patients diagnosed with MM and DLBCL from 2018 to 2022 using two distinct approaches for identifying TCL cases, which we describe below. A total of 113 unique TCL diagnosis codes, listed in the appendix, were used.
To estimate TCL prevalence in MM and DLBCL patients, we first assessed the number of patients who had at-least 5 billable interactions with a healthcare provider attached to a TCL diagnosis code in the year of MM/DLBCL diagnosis or after, through 2023. Patients with a TCL diagnosis preceding their initial MM or DLBCL diagnosis were included, provided they had at least 5 TCL-related billable interactions in the year of their MM or DLBCL diagnosis or after. Our second approach assessed the number of patients who received at least 1 TCL diagnosis code any time after the initial MM or DLBCL claim was received, through 2023. Patients with a TCL diagnosis preceding their initial MM or DLBCL diagnosis were included, provided they had at least 1 TCL-related billable interaction after their MM or DLBCL diagnosis.
To estimate the incidence of newly diagnosed TCL after a MM or DLBCL diagnosis, we first assessed the number of patients who received a TCL diagnosis after their MM or DLBCL diagnosis through 2023 and who had at-least 5 billable interactions with a healthcare provider attached to a TCL diagnosis code. Patients with a TCL diagnosis preceding their initial MM or DLBCL diagnosis were excluded. Our second approach assessed the number of patients who received at least 1 TCL diagnosis code any time after the initial MM or DLBCL claim was received through 2023. Similarly, patients with a TCL diagnosis preceding the MM or DLBCL diagnosis were excluded.
Since all methodologies look at TCL diagnosis in the years following the initial MM or DLBCL diagnosis, we should expect that the prevalence and incidence may be underestimated in the outer years of our analysis (i.e., for a patient with an initial DLBCL diagnosis in 2018, we look at TCL billable interactions between 2018-2023, whereas for a patient with an initial DLBCL diagnosis in 2022, we only look at a 2 year timeframe for TCL billable interactions).
Results
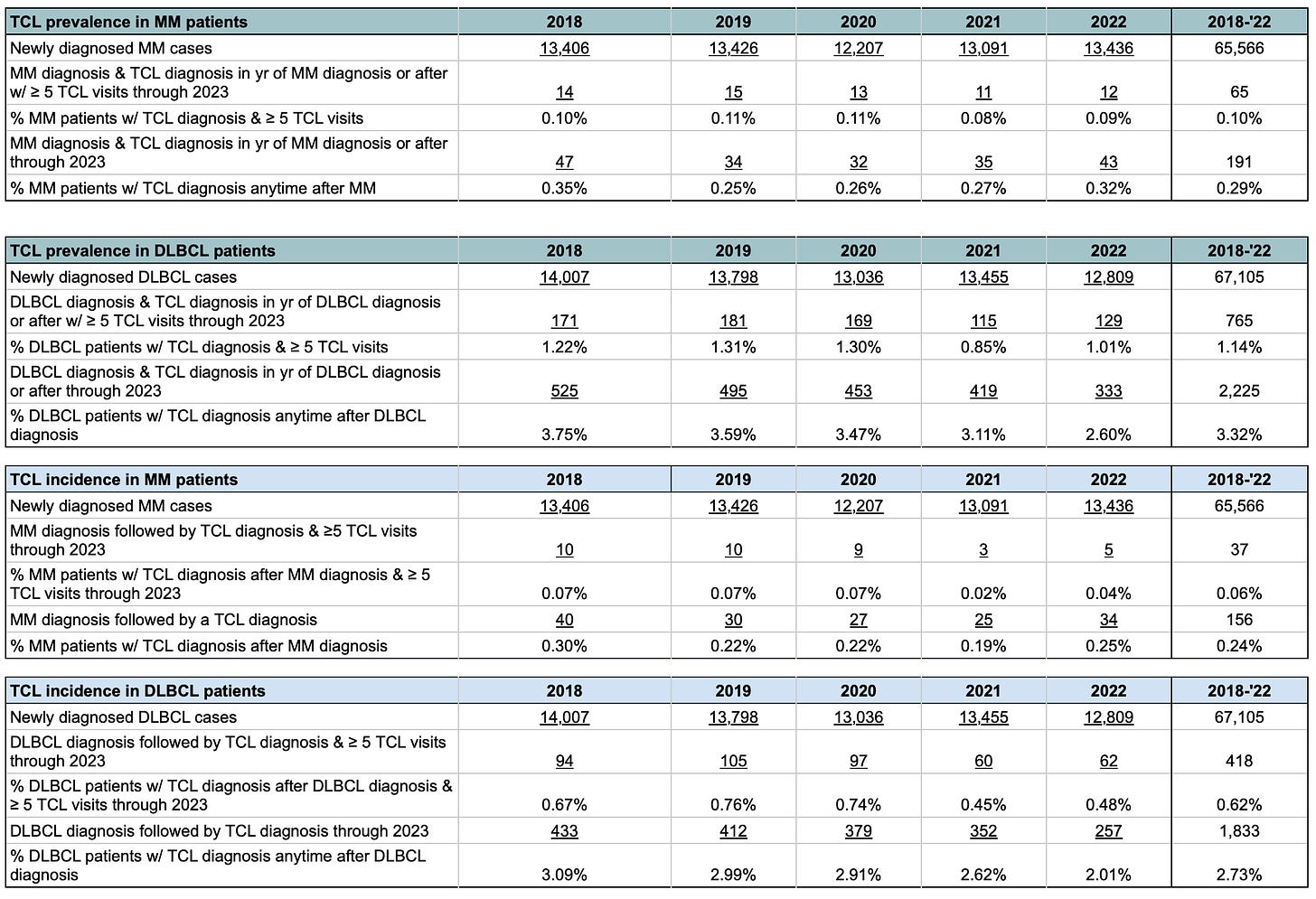
For MM patients, we saw TCL prevalence of 0.10%, or 99 per 100,000 MM patients newly diagnosed over a 5-year period, using the methodology that required 5 or more billable TCL interactions. When we did the same analysis using the methodology that required only 1 or more billable TCL interactions, the rate increased to 0.29%, which translates to 291 per 100,000 MM patients. Not surprisingly, by omitting the multi-claim requirement, the potential to capture incorrectly diagnosed TCL patients increased and may have led to an overestimation of prevalence.
We saw TCL incidence of 0.06%, or 56 cases per 100,000 MM patients, using the methodology that required 5 or more billable TCL interactions. When we did the same analysis using the methodology that required only 1 or more billable TCL interactions, the incidence rate increased to 0.24%, which translates to 238 cases per 100,000 MM patients.
For DLBCL, we saw TCL prevalence of 1.14%, or 1,140 per 100,000 DLBCL patients, using the methodology that required 5 or more billable TCL interactions. When we did the same analysis using the methodology that required only 1 or more billable TCL interactions, the annual rate increased to 3.32%, which translates to 3,316 per 100,000 DLBCL patients.
We saw TCL incidence of 0.62%, or 623 cases per 100,000 DLBCL patients, using the methodology that required 5 or more billable TCL interactions. When we did the same analysis using the methodology that required only 1 or more billable TCL interactions, the incidence rate increased to 2.73% or 2,732 cases per 100,000 DLBCL patients.
This compares favorably to a rate of ~81 reported TCL cases per 100,000 patients treated with BCMA or CD19 CAR-T therapies.
Discussion
Our analysis of a large RWE database suggests that the background incidence for TCL among patients diagnosed with MM or DLBCL is not substantially different from that reported by the FDA in patients who have received prior CAR-T treatments. We should note that we don’t have a sound methodology to compare incidence in newly diagnosed MM and DLBCL patients as compared with advanced disease patients, who have different survival statistics and follow up. But we can attest that the background incidence of TCL is significant in patients who have not received CAR-T. We believe it is important to provide this context so that we better understand the incremental risk of developing TCL among those being treated with CAR-T therapy. This question is of high relevance given that there's a theoretical possibility of CAR-T vectors becoming oncogenic. Lymphoma originating from CAR-T cells produced with the piggyBac transposon system has been seen in 2 patients.[2] At least one case report has identified a CAR+ case of T-cell lymphoma, although a potential contributory role of the CAR insertion was unclear as the patient may have had an existing lymphoma clone prior to receiving therapy.[3]
Occurrence of T cell lymphoma among patients with MM and DLBCL could be increased compared to the general population by several factors. First, it is possible that there are germline genetic susceptibilities that would make patients more prone to the development of other similar hematological malignancies. Second, another explanation could be that similar exposure may lead to stimulation of precursor states that ultimately lead to T cell malignancies. Third, with a primary neoplastic diagnosis additional surveillance imaging is routinely done. Lastly, it is also possible that some of the therapeutics used for the treatment of MM or DLBCL could ultimately lead to the generation of TCL. As an example, lenalidomide and melphalan, common medications used for the treatment of MM have been associated with secondary malignancies including myelodysplasia and acute leukemia.[4, 5] Amongst the leukemia diagnosed, acute lymphocytic leukemia (ALL) has been reported.[6] In addition, IKZF1, a target for CRBN mediated degradation via lenalidomide, is a tumor suppressor for T-ALL.[7, 8] In a large meta-analysis the risk of secondary malignancies after R-CHOP chemotherapy has been estimated as high as 10-15%, and higher if radiation was used. We would imagine that it is possible in some cases that therapies could lead to the development of TCL.
Our data suggest a slightly higher incidence of TCL in patients with DLBCL as compared to MM. More frequent surveillance imaging is done in patients with lymphoma than for myeloma. One could imagine that the lymphoma susceptibility mechanisms could be more likely to lead to a secondary TCL in the DLBCL patients. One study shows bidirectional (B cell to T cell and vice versa) increased risk for development of lymphoma among patients with a primary diagnosis. The use of RWE data makes it plausible that either misdiagnosis or miscoding results in this reported higher incidence. This hypothesis is further supported by the fact that a more stringent inclusion criteria (5 visits coded for TCL) resulted in lower prevalence numbers.
It is possible that we are underestimating risk due to the shorter follow up for BCMA CAR-T and the high disease mortality after such treatments for MM.
An additional warning was subsequently made by the FDA regarding the possibility of myeloid malignancies increased frequency in patients treated with CAR-T for MM. The scope of such discussion is beyond the scope of the data presented here. Such myeloid malignancies have also been reported in up to 5% of patients with lymphoma treated with CAR-Ts and deserve further study. The implications of prior therapy in such cases also remain.
Additional follow-up will be needed. We believe this information should be used as evidence not to stop the development and the use of CAR-Ts for MM or DLBCL for patients who so desperately need them. It will also be important to monitor whether a different CAR-T vector will have a different risk of transformation to TCL.
Addendum
This is the data from which we generated our results:
Copyright: ©️ 2024 Komodo Health, Inc., all rights reserved.
Disclosures
RF
Consulting: AbbVie, Adaptive Biotechnologies, AMGEN, AstraZeneca, Bayer, Binding Site, BMS (Celgene), Millenium Takeda, Janssen, Juno, Kite, Merck, Pfizer, Pharmacyclics, RA Capital Management, Regeneron, Sanofi.
Scientific Advisory Boards: Adaptive Biotechnologies, Caris Life Sciences, Oncotracker.
Board of Directors: Antegene (for profit)
Patent for FISH in MM - ~$2000/year
ER
Consultancy/Speaker: AbbVie, Sanofi, Janssen.
SC
Honoraria (Advisory board): Sanofi, GSK, Janssen, Institutional Research Funding: Abbvie, C4 Therapeutics, Janssen, CARSgen
AR
Honoraria from Curio Science OncLive and RMEI for educational events.
Appendix
Type of Code
Count
DLBCL Drug Codes
212
MM Drug Codes
161
Multiple Myeloma ICD10
8
T cell Lymphoma ICD10
113
B Cell Lymphoma ICD10
33
APPENDIX
Type of Code
Original code
Description
DLBCL Drug Codes
NDC:43598066011
1 ml cyclophosphamide 500 mg/ml injection
DLBCL Drug Codes
NDC:00069303120
10 ml doxorubicin hydrochloride 2 mg/ml injection
DLBCL Drug Codes
NDC:67457039400
10 ml doxorubicin hydrochloride 2 mg/ml injection
DLBCL Drug Codes
NDC:67457039410
10 ml doxorubicin hydrochloride 2 mg/ml injection
DLBCL Drug Codes
NDC:143908501
10 ml doxorubicin hydrochloride 2 mg/ml injection
DLBCL Drug Codes
NDC:69401510
10 ml doxorubicin hydrochloride 2 mg/ml injection
DLBCL Drug Codes
NDC:143954801
10 ml doxorubicin hydrochloride 2 mg/ml injection [adriamycin]
DLBCL Drug Codes
NDC:143954810
10 ml doxorubicin hydrochloride 2 mg/ml injection [adriamycin]
DLBCL Drug Codes
NDC:70710153001
10 ml doxorubicin hydrochloride liposome 2 mg/ml injection
DLBCL Drug Codes
NDC:68001034536
10 ml doxorubicin hydrochloride liposome 2 mg/ml injection
DLBCL Drug Codes
NDC:47335004940
10 ml doxorubicin hydrochloride liposome 2 mg/ml injection
DLBCL Drug Codes
NDC:43598068235
10 ml doxorubicin hydrochloride liposome 2 mg/ml injection
DLBCL Drug Codes
NDC:43598028335
10 ml doxorubicin hydrochloride liposome 2 mg/ml injection
DLBCL Drug Codes
NDC:16714074201
10 ml doxorubicin hydrochloride liposome 2 mg/ml injection
DLBCL Drug Codes
NDC:72603010301
10 ml doxorubicin hydrochloride liposome 2 mg/ml injection
DLBCL Drug Codes
NDC:338008001
10 ml doxorubicin hydrochloride liposome 2 mg/ml injection
DLBCL Drug Codes
NDC:68001049236
10 ml doxorubicin hydrochloride liposome 2 mg/ml injection
DLBCL Drug Codes
NDC:59676096001
10 ml doxorubicin hydrochloride liposome 2 mg/ml injection [doxil]
DLBCL Drug Codes
NDC:00338006301
10 ml doxorubicin hydrochloride liposome 2 mg/ml injection [doxil]
DLBCL Drug Codes
NDC:338006301
10 ml doxorubicin hydrochloride liposome 2 mg/ml injection [doxil]
DLBCL Drug Codes
NDC:47335008250
10 ml doxorubicin hydrochloride liposome 2 mg/ml injection [lipodox]
DLBCL Drug Codes
NDC:50242005121
10 ml rituximab 10 mg/ml injection [rituxan]
DLBCL Drug Codes
NDC:50242005110
10 ml rituximab 10 mg/ml injection [rituxan]
DLBCL Drug Codes
NDC:63459010310
10 ml rituximab-abbs 10 mg/ml injection [truxima]
DLBCL Drug Codes
NDC:55513022401
10 ml rituximab-arrx 10 mg/ml injection [riabni]
DLBCL Drug Codes
NDC:00069023801
10 ml rituximab-pvvr 10 mg/ml injection [ruxience]
DLBCL Drug Codes
NDC:69023801
10 ml rituximab-pvvr 10 mg/ml injection [ruxience]
DLBCL Drug Codes
NDC:50242010886
11.7 ml hyaluronidase, human recombinant 2000 unt/ml / rituximab 120 mg/ml injection [rituxan hycela]
DLBCL Drug Codes
NDC:50242010801
11.7 ml hyaluronidase, human recombinant 2000 unt/ml / rituximab 120 mg/ml injection [rituxan hycela]
DLBCL Drug Codes
NDC:50242010901
13.4 ml hyaluronidase, human recombinant 2000 unt/ml / rituximab 120 mg/ml injection [rituxan hycela]
DLBCL Drug Codes
NDC:43598066111
2 ml cyclophosphamide 500 mg/ml injection
DLBCL Drug Codes
NDC:55150027001
2.5 ml cyclophosphamide 200 mg/ml injection
DLBCL Drug Codes
NDC:63323088330
25 ml doxorubicin hydrochloride 2 mg/ml injection
DLBCL Drug Codes
NDC:25021020725
25 ml doxorubicin hydrochloride 2 mg/ml injection
DLBCL Drug Codes
NDC:00143908601
25 ml doxorubicin hydrochloride 2 mg/ml injection
DLBCL Drug Codes
NDC:00069403201
25 ml doxorubicin hydrochloride 2 mg/ml injection
DLBCL Drug Codes
NDC:00069402625
25 ml doxorubicin hydrochloride 2 mg/ml injection
DLBCL Drug Codes
NDC:00069303220
25 ml doxorubicin hydrochloride 2 mg/ml injection
DLBCL Drug Codes
NDC:62756082640
25 ml doxorubicin hydrochloride 2 mg/ml injection
DLBCL Drug Codes
NDC:143908601
25 ml doxorubicin hydrochloride 2 mg/ml injection
DLBCL Drug Codes
NDC:69402625
25 ml doxorubicin hydrochloride 2 mg/ml injection
DLBCL Drug Codes
NDC:00143954701
25 ml doxorubicin hydrochloride 2 mg/ml injection [adriamycin]
DLBCL Drug Codes
NDC:143954701
25 ml doxorubicin hydrochloride 2 mg/ml injection [adriamycin]
DLBCL Drug Codes
NDC:47335005040
25 ml doxorubicin hydrochloride liposome 2 mg/ml injection
DLBCL Drug Codes
NDC:43598054125
25 ml doxorubicin hydrochloride liposome 2 mg/ml injection
DLBCL Drug Codes
NDC:16714085601
25 ml doxorubicin hydrochloride liposome 2 mg/ml injection
DLBCL Drug Codes
NDC:68001034526
25 ml doxorubicin hydrochloride liposome 2 mg/ml injection
DLBCL Drug Codes
NDC:72603020001
25 ml doxorubicin hydrochloride liposome 2 mg/ml injection
DLBCL Drug Codes
NDC:338008601
25 ml doxorubicin hydrochloride liposome 2 mg/ml injection
DLBCL Drug Codes
NDC:70710153101
25 ml doxorubicin hydrochloride liposome 2 mg/ml injection
DLBCL Drug Codes
NDC:68001049326
25 ml doxorubicin hydrochloride liposome 2 mg/ml injection
DLBCL Drug Codes
NDC:43598068325
25 ml doxorubicin hydrochloride liposome 2 mg/ml injection
DLBCL Drug Codes
NDC:59676096002
25 ml doxorubicin hydrochloride liposome 2 mg/ml injection [doxil]
DLBCL Drug Codes
NDC:00338006701
25 ml doxorubicin hydrochloride liposome 2 mg/ml injection [doxil]
DLBCL Drug Codes
NDC:338006701
25 ml doxorubicin hydrochloride liposome 2 mg/ml injection [doxil]
DLBCL Drug Codes
NDC:47335008350
25 ml doxorubicin hydrochloride liposome 2 mg/ml injection [lipodox]
DLBCL Drug Codes
NDC:43598066211
4 ml cyclophosphamide 500 mg/ml injection
DLBCL Drug Codes
NDC:55150027101
5 ml cyclophosphamide 200 mg/ml injection
DLBCL Drug Codes
NDC:63323088305
5 ml doxorubicin hydrochloride 2 mg/ml injection
DLBCL Drug Codes
NDC:00703504303
5 ml doxorubicin hydrochloride 2 mg/ml injection
DLBCL Drug Codes
NDC:67457039300
5 ml doxorubicin hydrochloride 2 mg/ml injection
DLBCL Drug Codes
NDC:25021020705
5 ml doxorubicin hydrochloride 2 mg/ml injection
DLBCL Drug Codes
NDC:143908401
5 ml doxorubicin hydrochloride 2 mg/ml injection
DLBCL Drug Codes
NDC:69400405
5 ml doxorubicin hydrochloride 2 mg/ml injection
DLBCL Drug Codes
NDC:703504301
5 ml doxorubicin hydrochloride 2 mg/ml injection
DLBCL Drug Codes
NDC:143954910
5 ml doxorubicin hydrochloride 2 mg/ml injection [adriamycin]
DLBCL Drug Codes
NDC:143954901
5 ml doxorubicin hydrochloride 2 mg/ml injection [adriamycin]
DLBCL Drug Codes
NDC:50242005306
50 ml rituximab 10 mg/ml injection [rituxan]
DLBCL Drug Codes
NDC:63459010450
50 ml rituximab-abbs 10 mg/ml injection [truxima]
DLBCL Drug Codes
NDC:55513032601
50 ml rituximab-arrx 10 mg/ml injection [riabni]
DLBCL Drug Codes
NDC:00069024901
50 ml rituximab-pvvr 10 mg/ml injection [ruxience]
DLBCL Drug Codes
NDC:69024901
50 ml rituximab-pvvr 10 mg/ml injection [ruxience]
DLBCL Drug Codes
PDM3237
cyclophosphamide
DLBCL Drug Codes
NDC:72603032601
cyclophosphamide 1000 mg injection
DLBCL Drug Codes
NDC:70121123901
cyclophosphamide 1000 mg injection
DLBCL Drug Codes
NDC:68001044327
cyclophosphamide 1000 mg injection
DLBCL Drug Codes
NDC:68001037133
cyclophosphamide 1000 mg injection
DLBCL Drug Codes
NDC:16714085701
cyclophosphamide 1000 mg injection
DLBCL Drug Codes
NDC:10019095616
cyclophosphamide 1000 mg injection
DLBCL Drug Codes
NDC:10019095601
cyclophosphamide 1000 mg injection
DLBCL Drug Codes
NDC:10019094450
cyclophosphamide 1000 mg injection
DLBCL Drug Codes
NDC:10019094401
cyclophosphamide 1000 mg injection
DLBCL Drug Codes
NDC:10019093950
cyclophosphamide 1000 mg injection
DLBCL Drug Codes
NDC:10019093901
cyclophosphamide 1000 mg injection
DLBCL Drug Codes
NDC:00781324494
cyclophosphamide 1000 mg injection
DLBCL Drug Codes
NDC:781324494
cyclophosphamide 1000 mg injection
DLBCL Drug Codes
NDC:72572008501
cyclophosphamide 1000 mg injection
DLBCL Drug Codes
NDC:68001037132
cyclophosphamide 1000 mg injection
DLBCL Drug Codes
NDC:70860021805
cyclophosphamide 200 mg/ml injectable solution
DLBCL Drug Codes
NDC:70860021803
cyclophosphamide 200 mg/ml injectable solution
DLBCL Drug Codes
NDC:50742052110
cyclophosphamide 200 mg/ml injectable solution
DLBCL Drug Codes
NDC:50742052005
cyclophosphamide 200 mg/ml injectable solution
DLBCL Drug Codes
NDC:50742051902
cyclophosphamide 200 mg/ml injectable solution
DLBCL Drug Codes
NDC:51407074802
cyclophosphamide 200 mg/ml injectable solution
DLBCL Drug Codes
NDC:51407074905
cyclophosphamide 200 mg/ml injectable solution
DLBCL Drug Codes
NDC:51407075010
cyclophosphamide 200 mg/ml injectable solution
DLBCL Drug Codes
NDC:70121124001
cyclophosphamide 2000 mg injection
DLBCL Drug Codes
NDC:16714085801
cyclophosphamide 2000 mg injection
DLBCL Drug Codes
NDC:10019095711
cyclophosphamide 2000 mg injection
DLBCL Drug Codes
NDC:10019095701
cyclophosphamide 2000 mg injection
DLBCL Drug Codes
NDC:10019094210
cyclophosphamide 2000 mg injection
DLBCL Drug Codes
NDC:10019094201
cyclophosphamide 2000 mg injection
DLBCL Drug Codes
NDC:00781325594
cyclophosphamide 2000 mg injection
DLBCL Drug Codes
NDC:781325594
cyclophosphamide 2000 mg injection
DLBCL Drug Codes
NDC:68001044432
cyclophosphamide 2000 mg injection
DLBCL Drug Codes
NDC:72572008301
cyclophosphamide 2000 mg injection
DLBCL Drug Codes
NDC:68001037232
cyclophosphamide 2000 mg injection
DLBCL Drug Codes
NDC:69097051607
cyclophosphamide 25 mg oral capsule
DLBCL Drug Codes
NDC:54879002101
cyclophosphamide 25 mg oral capsule
DLBCL Drug Codes
NDC:43975030710
cyclophosphamide 25 mg oral capsule
DLBCL Drug Codes
NDC:00054038225
cyclophosphamide 25 mg oral capsule
DLBCL Drug Codes
NDC:54038225
cyclophosphamide 25 mg oral capsule
DLBCL Drug Codes
NDC:62559093001
cyclophosphamide 25 mg oral capsule
DLBCL Drug Codes
NDC:62332061831
cyclophosphamide 25 mg oral capsule
DLBCL Drug Codes
NDC:00054412925
cyclophosphamide 25 mg oral tablet
DLBCL Drug Codes
NDC:10019098201
cyclophosphamide 25 mg oral tablet
DLBCL Drug Codes
NDC:54412925
cyclophosphamide 25 mg oral tablet
DLBCL Drug Codes
NDC:54808925
cyclophosphamide 25 mg oral tablet
DLBCL Drug Codes
NDC:15050401
cyclophosphamide 25 mg oral tablet [cytoxan]
DLBCL Drug Codes
NDC:69097051707
cyclophosphamide 50 mg oral capsule
DLBCL Drug Codes
NDC:62559093101
cyclophosphamide 50 mg oral capsule
DLBCL Drug Codes
NDC:54879002201
cyclophosphamide 50 mg oral capsule
DLBCL Drug Codes
NDC:43975030810
cyclophosphamide 50 mg oral capsule
DLBCL Drug Codes
NDC:00054038325
cyclophosphamide 50 mg oral capsule
DLBCL Drug Codes
NDC:54038325
cyclophosphamide 50 mg oral capsule
DLBCL Drug Codes
NDC:62332061931
cyclophosphamide 50 mg oral capsule
DLBCL Drug Codes
NDC:10019098401
cyclophosphamide 50 mg oral tablet
DLBCL Drug Codes
NDC:00054413025
cyclophosphamide 50 mg oral tablet
DLBCL Drug Codes
NDC:54413025
cyclophosphamide 50 mg oral tablet
DLBCL Drug Codes
NDC:54813025
cyclophosphamide 50 mg oral tablet
DLBCL Drug Codes
NDC:15050301
cyclophosphamide 50 mg oral tablet [cytoxan]
DLBCL Drug Codes
NDC:70121123801
cyclophosphamide 500 mg injection
DLBCL Drug Codes
NDC:68001044226
cyclophosphamide 500 mg injection
DLBCL Drug Codes
NDC:68001037024
cyclophosphamide 500 mg injection
DLBCL Drug Codes
NDC:16714085901
cyclophosphamide 500 mg injection
DLBCL Drug Codes
NDC:10019095550
cyclophosphamide 500 mg injection
DLBCL Drug Codes
NDC:10019095501
cyclophosphamide 500 mg injection
DLBCL Drug Codes
NDC:10019094301
cyclophosphamide 500 mg injection
DLBCL Drug Codes
NDC:10019093825
cyclophosphamide 500 mg injection
DLBCL Drug Codes
NDC:10019093801
cyclophosphamide 500 mg injection
DLBCL Drug Codes
NDC:10019093501
cyclophosphamide 500 mg injection
DLBCL Drug Codes
NDC:00781323394
cyclophosphamide 500 mg injection
DLBCL Drug Codes
NDC:781323394
cyclophosphamide 500 mg injection
DLBCL Drug Codes
NDC:72603010401
cyclophosphamide 500 mg injection
DLBCL Drug Codes
NDC:72572008701
cyclophosphamide 500 mg injection
DLBCL Drug Codes
NDC:68001037027
cyclophosphamide 500 mg injection
DLBCL Drug Codes
PROC:J9070
Cyclophosphamide, 100 mg
DLBCL Drug Codes
PROC:J8530
Cyclophosphamide; oral, 25 mg
DLBCL Drug Codes
PDM4754
doxorubicin hydrochloride
DLBCL Drug Codes
NDC:143909201
doxorubicin hydrochloride 10 mg injection
DLBCL Drug Codes
NDC:143927501
doxorubicin hydrochloride 10 mg injection [adriamycin]
DLBCL Drug Codes
NDC:70860020851
doxorubicin hydrochloride 2 mg/ml injectable solution
DLBCL Drug Codes
NDC:63323088310
doxorubicin hydrochloride 2 mg/ml injectable solution
DLBCL Drug Codes
NDC:63323010161
doxorubicin hydrochloride 2 mg/ml injectable solution
DLBCL Drug Codes
NDC:45963073368
doxorubicin hydrochloride 2 mg/ml injectable solution
DLBCL Drug Codes
NDC:45963073360
doxorubicin hydrochloride 2 mg/ml injectable solution
DLBCL Drug Codes
NDC:25021020751
doxorubicin hydrochloride 2 mg/ml injectable solution
DLBCL Drug Codes
NDC:16714000101
doxorubicin hydrochloride 2 mg/ml injectable solution
DLBCL Drug Codes
NDC:00703504601
doxorubicin hydrochloride 2 mg/ml injectable solution
DLBCL Drug Codes
NDC:00703504001
doxorubicin hydrochloride 2 mg/ml injectable solution
DLBCL Drug Codes
NDC:00143908701
doxorubicin hydrochloride 2 mg/ml injectable solution
DLBCL Drug Codes
NDC:00069403701
doxorubicin hydrochloride 2 mg/ml injectable solution
DLBCL Drug Codes
NDC:00069403401
doxorubicin hydrochloride 2 mg/ml injectable solution
DLBCL Drug Codes
NDC:00069403001
doxorubicin hydrochloride 2 mg/ml injectable solution
DLBCL Drug Codes
NDC:00069303420
doxorubicin hydrochloride 2 mg/ml injectable solution
DLBCL Drug Codes
NDC:00069303320
doxorubicin hydrochloride 2 mg/ml injectable solution
DLBCL Drug Codes
NDC:00069303020
doxorubicin hydrochloride 2 mg/ml injectable solution
DLBCL Drug Codes
NDC:00069017101
doxorubicin hydrochloride 2 mg/ml injectable solution
DLBCL Drug Codes
NDC:45963073355
doxorubicin hydrochloride 2 mg/ml injectable solution
DLBCL Drug Codes
NDC:67457047810
doxorubicin hydrochloride 2 mg/ml injectable solution
DLBCL Drug Codes
NDC:67457039610
doxorubicin hydrochloride 2 mg/ml injectable solution
DLBCL Drug Codes
NDC:67457039525
doxorubicin hydrochloride 2 mg/ml injectable solution
DLBCL Drug Codes
NDC:67457039354
doxorubicin hydrochloride 2 mg/ml injectable solution
DLBCL Drug Codes
NDC:62756082740
doxorubicin hydrochloride 2 mg/ml injectable solution
DLBCL Drug Codes
NDC:143908701
doxorubicin hydrochloride 2 mg/ml injectable solution
DLBCL Drug Codes
NDC:69303320
doxorubicin hydrochloride 2 mg/ml injectable solution
DLBCL Drug Codes
NDC:69403701
doxorubicin hydrochloride 2 mg/ml injectable solution
DLBCL Drug Codes
NDC:55390024301
doxorubicin hydrochloride 2 mg/ml injectable solution
DLBCL Drug Codes
NDC:55390024110
doxorubicin hydrochloride 2 mg/ml injectable solution
DLBCL Drug Codes
NDC:55390024701
doxorubicin hydrochloride 2 mg/ml injectable solution
DLBCL Drug Codes
NDC:55390024610
doxorubicin hydrochloride 2 mg/ml injectable solution
DLBCL Drug Codes
NDC:55390024510
doxorubicin hydrochloride 2 mg/ml injectable solution
DLBCL Drug Codes
NDC:55390024801
doxorubicin hydrochloride 2 mg/ml injectable solution
DLBCL Drug Codes
NDC:53150032001
doxorubicin hydrochloride 2 mg/ml injectable solution
DLBCL Drug Codes
NDC:69017001
doxorubicin hydrochloride 2 mg/ml injectable solution
DLBCL Drug Codes
NDC:00143954601
doxorubicin hydrochloride 2 mg/ml injectable solution [adriamycin]
DLBCL Drug Codes
NDC:00143937201
doxorubicin hydrochloride 2 mg/ml injectable solution [adriamycin]
DLBCL Drug Codes
NDC:143954601
doxorubicin hydrochloride 2 mg/ml injectable solution [adriamycin]
DLBCL Drug Codes
NDC:67457043650
doxorubicin hydrochloride 50 mg injection
DLBCL Drug Codes
NDC:143909301
doxorubicin hydrochloride 50 mg injection
DLBCL Drug Codes
NDC:00143927701
doxorubicin hydrochloride 50 mg injection [adriamycin]
DLBCL Drug Codes
NDC:143927701
doxorubicin hydrochloride 50 mg injection [adriamycin]
DLBCL Drug Codes
PDM9416
doxorubicin hydrochloride liposome
DLBCL Drug Codes
PDM4870
hyaluronidase-human recombinant
DLBCL Drug Codes
PROC:J9000
Injection, doxorubicin hydrochloride, 10 mg
DLBCL Drug Codes
PROC:Q2049
Injection, doxorubicin hydrochloride, liposomal, imported lipodox, 10 mg
DLBCL Drug Codes
PROC:Q2050
Injection, doxorubicin hydrochloride, liposomal, not otherwise specified, 10 mg
DLBCL Drug Codes
PROC:J9311
Injection, rituximab 10 mg and hyaluronidase
DLBCL Drug Codes
PROC:C9467
Injection, rituximab and hyaluronidase, 10 mg
DLBCL Drug Codes
PROC:Q5115
Injection, rituximab-abbs, biosimilar, (truxima), 10 mg
DLBCL Drug Codes
PROC:Q5123
Injection, rituximab-arrx, biosimilar, (riabni), 10 mg
DLBCL Drug Codes
PROC:J9312
Injection, rituximab, 10 mg
DLBCL Drug Codes
PROC:J9310
Injection, rituximab, 100 mg
DLBCL Drug Codes
PDM1211
rituximab
DLBCL Drug Codes
PDM4926
rituximab-abbs
DLBCL Drug Codes
PDM4895
rituximab-arrx
DLBCL Drug Codes
PDM4898
rituximab-pvvr
DLBCL Drug Codes
NDC:55390023210
DOXORUBICIN HCL
DLBCL Drug Codes
NDC:55390023110
DOXORUBICIN HCL
DLBCL Drug Codes
NDC:15050241
CYCLOPHOSPHAMIDE
DLBCL Drug Codes
NDC:15050541
CYCLOPHOSPHAMIDE
DLBCL Drug Codes
NDC:15050641
CYCLOPHOSPHAMIDE
MM Drug Codes
NDC:72266024401
1.4 ml bortezomib 2.5 mg/ml injection
MM Drug Codes
NDC:70511016202
1.4 ml bortezomib 2.5 mg/ml injection
MM Drug Codes
NDC:57894050301
15 ml daratumumab-fihj 120 mg/ml / hyaluronidase-fihj 2000 unt/ml injection [darzalex faspro]
MM Drug Codes
NDC:57894050520
20 ml daratumumab 20 mg/ml injection [darzalex]
MM Drug Codes
NDC:57894050220
20 ml daratumumab 20 mg/ml injection [darzalex]
MM Drug Codes
NDC:72266024301
3.5 ml bortezomib 1 mg/ml injection
MM Drug Codes
NDC:70511016105
3.5 ml bortezomib 1 mg/ml injection
MM Drug Codes
NDC:57894050505
5 ml daratumumab 20 mg/ml injection [darzalex]
MM Drug Codes
NDC:57894050205
5 ml daratumumab 20 mg/ml injection [darzalex]
MM Drug Codes
PDM1379
bortezomib
MM Drug Codes
NDC:00409170401
bortezomib 1 mg injection
MM Drug Codes
NDC:409170401
bortezomib 1 mg injection
MM Drug Codes
NDC:00409170301
bortezomib 2.5 mg injection
MM Drug Codes
NDC:409170301
bortezomib 2.5 mg injection
MM Drug Codes
NDC:72205018301
bortezomib 3.5 mg injection
MM Drug Codes
NDC:71288011810
bortezomib 3.5 mg injection
MM Drug Codes
NDC:70860022510
bortezomib 3.5 mg injection
MM Drug Codes
NDC:70771170801
bortezomib 3.5 mg injection
MM Drug Codes
NDC:70710141101
bortezomib 3.5 mg injection
MM Drug Codes
NDC:68001054136
bortezomib 3.5 mg injection
MM Drug Codes
NDC:68001054036
bortezomib 3.5 mg injection
MM Drug Codes
NDC:68001053436
bortezomib 3.5 mg injection
MM Drug Codes
NDC:67184053001
bortezomib 3.5 mg injection
MM Drug Codes
NDC:63323082110
bortezomib 3.5 mg injection
MM Drug Codes
NDC:63323072110
bortezomib 3.5 mg injection
MM Drug Codes
NDC:60505605004
bortezomib 3.5 mg injection
MM Drug Codes
NDC:55150033701
bortezomib 3.5 mg injection
MM Drug Codes
NDC:51817058601
bortezomib 3.5 mg injection
MM Drug Codes
NDC:50742048401
bortezomib 3.5 mg injection
MM Drug Codes
NDC:43598086560
bortezomib 3.5 mg injection
MM Drug Codes
NDC:43598042660
bortezomib 3.5 mg injection
MM Drug Codes
NDC:25021024410
bortezomib 3.5 mg injection
MM Drug Codes
NDC:10019099101
bortezomib 3.5 mg injection
MM Drug Codes
NDC:00781325870
bortezomib 3.5 mg injection
MM Drug Codes
NDC:00409170001
bortezomib 3.5 mg injection
MM Drug Codes
NDC:00143909801
bortezomib 3.5 mg injection
MM Drug Codes
NDC:409170001
bortezomib 3.5 mg injection
MM Drug Codes
NDC:143909801
bortezomib 3.5 mg injection
MM Drug Codes
NDC:63020004904
bortezomib 3.5 mg injection [velcade]
MM Drug Codes
NDC:63020004903
bortezomib 3.5 mg injection [velcade]
MM Drug Codes
NDC:63020004902
bortezomib 3.5 mg injection [velcade]
MM Drug Codes
NDC:63020004901
bortezomib 3.5 mg injection [velcade]
MM Drug Codes
NDC:10130004901
bortezomib 3.5 mg injection [velcade]
MM Drug Codes
PDM37978
bortezomib boronic anhydride
MM Drug Codes
PDM3402
carfilzomib
MM Drug Codes
NDC:76075010301
carfilzomib 10 mg injection [kyprolis]
MM Drug Codes
NDC:76075010201
carfilzomib 30 mg injection [kyprolis]
MM Drug Codes
NDC:76075010101
carfilzomib 60 mg injection [kyprolis]
MM Drug Codes
PDM3530
daratumumab
MM Drug Codes
PDM9451
daratumumab-fihj
MM Drug Codes
PDM9452
hyaluronidase-fihj
MM Drug Codes
PROC:J9041
Injection, bortezomib, 0.1 mg
MM Drug Codes
PROC:J9044
Injection, bortezomib, not otherwise specified, 0.1 mg
MM Drug Codes
PROC:J9047
Injection, carfilzomib, 1 mg
MM Drug Codes
PROC:J9145
Injection, daratumumab, 10 mg
MM Drug Codes
PROC:C9476
Injection, daratumumab, 10 mg
MM Drug Codes
PDM928
lenalidomide
MM Drug Codes
NDC:00480124328
lenalidomide 10 mg oral capsule
MM Drug Codes
NDC:70710103207
lenalidomide 10 mg oral capsule
MM Drug Codes
NDC:69097038273
lenalidomide 10 mg oral capsule
MM Drug Codes
NDC:63304004327
lenalidomide 10 mg oral capsule
MM Drug Codes
NDC:60505453402
lenalidomide 10 mg oral capsule
MM Drug Codes
NDC:59651034428
lenalidomide 10 mg oral capsule
MM Drug Codes
NDC:480124328
lenalidomide 10 mg oral capsule
MM Drug Codes
NDC:47781048528
lenalidomide 10 mg oral capsule
MM Drug Codes
NDC:47781048501
lenalidomide 10 mg oral capsule
MM Drug Codes
NDC:43598051263
lenalidomide 10 mg oral capsule
MM Drug Codes
NDC:378193728
lenalidomide 10 mg oral capsule
MM Drug Codes
NDC:378193701
lenalidomide 10 mg oral capsule
MM Drug Codes
NDC:31722025928
lenalidomide 10 mg oral capsule
MM Drug Codes
NDC:66484241001
lenalidomide 10 mg oral capsule [revlimid]
MM Drug Codes
NDC:66484241000
lenalidomide 10 mg oral capsule [revlimid]
MM Drug Codes
NDC:63069031002
lenalidomide 10 mg oral capsule [revlimid]
MM Drug Codes
NDC:59572041030
lenalidomide 10 mg oral capsule [revlimid]
MM Drug Codes
NDC:59572041028
lenalidomide 10 mg oral capsule [revlimid]
MM Drug Codes
NDC:59572041000
lenalidomide 10 mg oral capsule [revlimid]
MM Drug Codes
NDC:43593041002
lenalidomide 10 mg oral capsule [revlimid]
MM Drug Codes
NDC:00480124421
lenalidomide 15 mg oral capsule
MM Drug Codes
NDC:70710103308
lenalidomide 15 mg oral capsule
MM Drug Codes
NDC:69097038381
lenalidomide 15 mg oral capsule
MM Drug Codes
NDC:63304004422
lenalidomide 15 mg oral capsule
MM Drug Codes
NDC:60505453502
lenalidomide 15 mg oral capsule
MM Drug Codes
NDC:59651034521
lenalidomide 15 mg oral capsule
MM Drug Codes
NDC:480124421
lenalidomide 15 mg oral capsule
MM Drug Codes
NDC:47781048677
lenalidomide 15 mg oral capsule
MM Drug Codes
NDC:47781048601
lenalidomide 15 mg oral capsule
MM Drug Codes
NDC:43598051321
lenalidomide 15 mg oral capsule
MM Drug Codes
NDC:378194121
lenalidomide 15 mg oral capsule
MM Drug Codes
NDC:378194101
lenalidomide 15 mg oral capsule
MM Drug Codes
NDC:31722026021
lenalidomide 15 mg oral capsule
MM Drug Codes
NDC:66484415201
lenalidomide 15 mg oral capsule [revlimid]
MM Drug Codes
NDC:66484415001
lenalidomide 15 mg oral capsule [revlimid]
MM Drug Codes
NDC:66484415000
lenalidomide 15 mg oral capsule [revlimid]
MM Drug Codes
NDC:63069031503
lenalidomide 15 mg oral capsule [revlimid]
MM Drug Codes
NDC:59572041521
lenalidomide 15 mg oral capsule [revlimid]
MM Drug Codes
NDC:59572041500
lenalidomide 15 mg oral capsule [revlimid]
MM Drug Codes
NDC:43593041502
lenalidomide 15 mg oral capsule [revlimid]
MM Drug Codes
NDC:69097060473
lenalidomide 2.5 mg oral capsule
MM Drug Codes
NDC:63304004127
lenalidomide 2.5 mg oral capsule
MM Drug Codes
NDC:60505453202
lenalidomide 2.5 mg oral capsule
MM Drug Codes
NDC:59651034228
lenalidomide 2.5 mg oral capsule
MM Drug Codes
NDC:480124128
lenalidomide 2.5 mg oral capsule
MM Drug Codes
NDC:47781048328
lenalidomide 2.5 mg oral capsule
MM Drug Codes
NDC:43598051663
lenalidomide 2.5 mg oral capsule
MM Drug Codes
NDC:378193528
lenalidomide 2.5 mg oral capsule
MM Drug Codes
NDC:378193501
lenalidomide 2.5 mg oral capsule
MM Drug Codes
NDC:31722025728
lenalidomide 2.5 mg oral capsule
MM Drug Codes
NDC:59572040228
lenalidomide 2.5 mg oral capsule [revlimid]
MM Drug Codes
NDC:59572040200
lenalidomide 2.5 mg oral capsule [revlimid]
MM Drug Codes
NDC:69097038481
lenalidomide 20 mg oral capsule
MM Drug Codes
NDC:63304004522
lenalidomide 20 mg oral capsule
MM Drug Codes
NDC:60505453602
lenalidomide 20 mg oral capsule
MM Drug Codes
NDC:59651034621
lenalidomide 20 mg oral capsule
MM Drug Codes
NDC:480124521
lenalidomide 20 mg oral capsule
MM Drug Codes
NDC:47781048777
lenalidomide 20 mg oral capsule
MM Drug Codes
NDC:43598051421
lenalidomide 20 mg oral capsule
MM Drug Codes
NDC:378194221
lenalidomide 20 mg oral capsule
MM Drug Codes
NDC:378194201
lenalidomide 20 mg oral capsule
MM Drug Codes
NDC:31722026121
lenalidomide 20 mg oral capsule
MM Drug Codes
NDC:59572042021
lenalidomide 20 mg oral capsule [revlimid]
MM Drug Codes
NDC:59572042000
lenalidomide 20 mg oral capsule [revlimid]
MM Drug Codes
NDC:00480124621
lenalidomide 25 mg oral capsule
MM Drug Codes
NDC:70710103508
lenalidomide 25 mg oral capsule
MM Drug Codes
NDC:69097038581
lenalidomide 25 mg oral capsule
MM Drug Codes
NDC:63304004622
lenalidomide 25 mg oral capsule
MM Drug Codes
NDC:60505453702
lenalidomide 25 mg oral capsule
MM Drug Codes
NDC:59651034721
lenalidomide 25 mg oral capsule
MM Drug Codes
NDC:480124621
lenalidomide 25 mg oral capsule
MM Drug Codes
NDC:47781048877
lenalidomide 25 mg oral capsule
MM Drug Codes
NDC:47781048801
lenalidomide 25 mg oral capsule
MM Drug Codes
NDC:43598051521
lenalidomide 25 mg oral capsule
MM Drug Codes
NDC:378194021
lenalidomide 25 mg oral capsule
MM Drug Codes
NDC:378194001
lenalidomide 25 mg oral capsule
MM Drug Codes
NDC:31722026221
lenalidomide 25 mg oral capsule
MM Drug Codes
NDC:66484425201
lenalidomide 25 mg oral capsule [revlimid]
MM Drug Codes
NDC:66484425000
lenalidomide 25 mg oral capsule [revlimid]
MM Drug Codes
NDC:63069032504
lenalidomide 25 mg oral capsule [revlimid]
MM Drug Codes
NDC:59572042525
lenalidomide 25 mg oral capsule [revlimid]
MM Drug Codes
NDC:59572042521
lenalidomide 25 mg oral capsule [revlimid]
MM Drug Codes
NDC:59572042500
lenalidomide 25 mg oral capsule [revlimid]
MM Drug Codes
NDC:43593042502
lenalidomide 25 mg oral capsule [revlimid]
MM Drug Codes
NDC:00480124228
lenalidomide 5 mg oral capsule
MM Drug Codes
NDC:70710103107
lenalidomide 5 mg oral capsule
MM Drug Codes
NDC:69097038173
lenalidomide 5 mg oral capsule
MM Drug Codes
NDC:63304004227
lenalidomide 5 mg oral capsule
MM Drug Codes
NDC:60505453302
lenalidomide 5 mg oral capsule
MM Drug Codes
NDC:59651034328
lenalidomide 5 mg oral capsule
MM Drug Codes
NDC:480124228
lenalidomide 5 mg oral capsule
MM Drug Codes
NDC:47781048428
lenalidomide 5 mg oral capsule
MM Drug Codes
NDC:47781048401
lenalidomide 5 mg oral capsule
MM Drug Codes
NDC:43598051163
lenalidomide 5 mg oral capsule
MM Drug Codes
NDC:378193628
lenalidomide 5 mg oral capsule
MM Drug Codes
NDC:378193601
lenalidomide 5 mg oral capsule
MM Drug Codes
NDC:31722025828
lenalidomide 5 mg oral capsule
MM Drug Codes
NDC:66484240501
lenalidomide 5 mg oral capsule [revlimid]
MM Drug Codes
NDC:66484240500
lenalidomide 5 mg oral capsule [revlimid]
MM Drug Codes
NDC:63069030501
lenalidomide 5 mg oral capsule [revlimid]
MM Drug Codes
NDC:59572040530
lenalidomide 5 mg oral capsule [revlimid]
MM Drug Codes
NDC:59572040528
lenalidomide 5 mg oral capsule [revlimid]
MM Drug Codes
NDC:59572040500
lenalidomide 5 mg oral capsule [revlimid]
MM Drug Codes
NDC:43593040502
lenalidomide 5 mg oral capsule [revlimid]
Multiple Myeloma ICD10
C90.0
C90.0 Multiple myeloma
Multiple Myeloma ICD10
C90.00
C90.00 Multiple myeloma not having achieved remission
Multiple Myeloma ICD10
C90.01
C90.01 Multiple myeloma in remission
Multiple Myeloma ICD10
C90.02
C90.02 Multiple myeloma in relapse
Multiple Myeloma ICD10
C90.1
C90.1 Plasma cell leukemia
Multiple Myeloma ICD10
C90.10
C90.10 Plasma cell leukemia not having achieved remission
Multiple Myeloma ICD10
C90.11
C90.11 Plasma cell leukemia in remission
Multiple Myeloma ICD10
C90.12
C90.12 Plasma cell leukemia in relapse
T cell Lymphoma ICD10
C91
Lymphoid leukemia
T cell Lymphoma ICD10
C9161
Prolymphocytic leukemia of T-cell type, in remission.
T cell Lymphoma ICD10
C9162
Prolymphocytic leukemia of T-cell type, in relapse
T cell Lymphoma ICD10
Peripheral T cell lymphoma, lymph nodes of axilla and upper limb
T cell Lymphoma ICD10
Peripheral T cell lymphoma, unspecified site, extranodal and solid organ sites
T cell Lymphoma ICD10
Peripheral T cell lymphoma, lymph nodes of head, face, and neck
T cell Lymphoma ICD10
Peripheral T cell lymphoma, intrathoracic lymph nodes
T cell Lymphoma ICD10
Peripheral T cell lymphoma, intra-abdominal lymph nodes
T cell Lymphoma ICD10
Peripheral T cell lymphoma, intrapelvic lymph nodes
T cell Lymphoma ICD10
Peripheral T cell lymphoma, lymph nodes of multiple sites
T cell Lymphoma ICD10
Peripheral T cell lymphoma, lymph nodes of inguinal region and lower limb
T cell Lymphoma ICD10
Peripheral T cell lymphoma, spleen
T cell Lymphoma ICD10
T cell Lymphoma ICD10
Adult T-cell lymphoma/leukemia (HTLV-1-associated) not having achieved remission
T cell Lymphoma ICD10
Other mature T/NK-cell lymphomas, intrathoracic lymph nodes
T cell Lymphoma ICD10
Adult T-cell lymphoma/leukemia (HTLV-1-associated), in relapse
T cell Lymphoma ICD10
Adult T-cell lymphoma/leukemia (HTLV-1-associated), in remission
T cell Lymphoma ICD10
Peripheral T-cell lymphoma, not classified, intrapelvic lymph nodes
T cell Lymphoma ICD10
Mature T/NK-cell lymphomas, unspecified, lymph nodes of head, face, and neck
T cell Lymphoma ICD10
Mature T/NK-cell lymphomas, unspecified, unspecified site
T cell Lymphoma ICD10
Mature T/NK-cell lymphomas, unspecified, intra-abdominal lymph nodes
T cell Lymphoma ICD10
Mature T/NK-cell lymphomas, unspecified, intrathoracic lymph nodes
T cell Lymphoma ICD10
Mature T/NK-cell lymphomas, unspecified, lymph nodes of inguinal region and lower limb
T cell Lymphoma ICD10
Mature T/NK-cell lymphomas, unspecified, lymph nodes of axilla and upper limb
T cell Lymphoma ICD10
Mature T/NK-cell lymphomas, unspecified, spleen
T cell Lymphoma ICD10
Mature T/NK-cell lymphomas, unspecified, intrapelvic lymph nodes
T cell Lymphoma ICD10
Mature T/NK-cell lymphomas, unspecified, extranodal and solid organ sites
T cell Lymphoma ICD10
Mature T/NK-cell lymphomas, unspecified, lymph nodes of multiple sites
T cell Lymphoma ICD10
Peripheral T-cell lymphoma, not classified, lymph nodes of head, face, and neck
T cell Lymphoma ICD10
Peripheral T-cell lymphoma, not classified, lymph nodes of multiple sites
T cell Lymphoma ICD10
Peripheral T-cell lymphoma, not classified, extranodal and solid organ sites
T cell Lymphoma ICD10
Peripheral T-cell lymphoma, not classified, unspecified site
T cell Lymphoma ICD10
Peripheral T-cell lymphoma, not classified, spleen
T cell Lymphoma ICD10
Peripheral T-cell lymphoma, not classified, lymph nodes of axilla and upper limb
T cell Lymphoma ICD10
Peripheral T-cell lymphoma, not classified, lymph nodes of inguinal region and lower limb
T cell Lymphoma ICD10
Peripheral T-cell lymphoma, not classified, intrathoracic lymph nodes
T cell Lymphoma ICD10
Peripheral T-cell lymphoma, not classified, intra-abdominal lymph nodes
T cell Lymphoma ICD10
Cutaneous T-cell lymphoma, unspecified
T cell Lymphoma ICD10
Other mature T/NK-cell lymphomas
T cell Lymphoma ICD10
Peripheral T-cell lymphoma, not classified
T cell Lymphoma ICD10
Mature T/NK-cell lymphomas, unspecified
T cell Lymphoma ICD10
Cutaneous T-cell lymphoma, unspecified, intrathoracic lymph nodes
T cell Lymphoma ICD10
Other specified types of T/NK-cell lymphoma
T cell Lymphoma ICD10
Other mature T/NK-cell lymphomas, unspecified site
T cell Lymphoma ICD10
Other mature T/NK-cell lymphomas, lymph nodes of head, face, and neck
T cell Lymphoma ICD10
Other mature T/NK-cell lymphomas, lymph nodes of multiple sites
T cell Lymphoma ICD10
Other mature T/NK-cell lymphomas, extranodal and solid organ sites
T cell Lymphoma ICD10
Other mature T/NK-cell lymphomas, intra-abdominal lymph nodes
T cell Lymphoma ICD10
Other mature T/NK-cell lymphomas, lymph nodes of axilla and upper limb
T cell Lymphoma ICD10
Other mature T/NK-cell lymphomas, lymph nodes of inguinal region and lower limb
T cell Lymphoma ICD10
Other mature T/NK-cell lymphomas, intrapelvic lymph nodes
T cell Lymphoma ICD10
Cutaneous T-cell lymphoma, unspecified lymph nodes of head, face, and neck
T cell Lymphoma ICD10
Cutaneous T-cell lymphoma, unspecified, unspecified site
T cell Lymphoma ICD10
Cutaneous T-cell lymphoma, unspecified, intra-abdominal lymph nodes
T cell Lymphoma ICD10
Cutaneous T-cell lymphoma, unspecified, lymph nodes of inguinal region and lower limb
T cell Lymphoma ICD10
Cutaneous T-cell lymphoma, unspecified, extranodal and solid organ sites
T cell Lymphoma ICD10
Cutaneous T-cell lymphoma, unspecified, lymph nodes of multiple sites
T cell Lymphoma ICD10
Cutaneous T-cell lymphoma, unspecified, lymph nodes of axilla and upper limb
T cell Lymphoma ICD10
Cutaneous T-cell lymphoma, unspecified, intrapelvic lymph nodes
T cell Lymphoma ICD10
Enteropathy-type (intestinal) T-cell lymphoma
T cell Lymphoma ICD10
Other mature T/NK-cell lymphomas, spleen
T cell Lymphoma ICD10
T cell Lymphoma ICD10
Extranodal NK/T-cell lymphoma, nasal type
T cell Lymphoma ICD10
Angioimmunoblastic T-cell lymphoma
T cell Lymphoma ICD10
Subcutaneous panniculitis-like T-cell lymphoma
T cell Lymphoma ICD10
T cell Lymphoma ICD10
Adult T-cell lymphoma/leukemia (HTLV-1-associated)
T cell Lymphoma ICD10
Cutaneous T-cell lymphoma, unspecified, spleen
T cell Lymphoma ICD10
C840
C84.0 Mycosis fungoides
T cell Lymphoma ICD10
C8400
C84.00 …… unspecified site
T cell Lymphoma ICD10
C8401
C84.01 …… lymph nodes of head, face, and neck
T cell Lymphoma ICD10
C8402
C84.02 …… intrathoracic lymph nodes
T cell Lymphoma ICD10
C8403
C84.03 …… intra-abdominal lymph nodes
T cell Lymphoma ICD10
C8404
C84.04 …… lymph nodes of axilla and upper limb
T cell Lymphoma ICD10
C8405
C84.05 …… lymph nodes of inguinal region and lower limb
T cell Lymphoma ICD10
C8406
C84.06 …… intrapelvic lymph nodes
T cell Lymphoma ICD10
C8407
C84.07 …… spleen
T cell Lymphoma ICD10
C8408
C84.08 …… lymph nodes of multiple sites
T cell Lymphoma ICD10
C8409
C84.09 …… extranodal and solid organ sites
T cell Lymphoma ICD10
C841
C84.1 Sézary disease
T cell Lymphoma ICD10
C8410
C84.10 …… unspecified site
T cell Lymphoma ICD10
C8411
C84.11 …… lymph nodes of head, face, and neck
T cell Lymphoma ICD10
C8412
C84.12 …… intrathoracic lymph nodes
T cell Lymphoma ICD10
C8413
C84.13 …… intra-abdominal lymph nodes
T cell Lymphoma ICD10
C8414
C84.14 …… lymph nodes of axilla and upper limb
T cell Lymphoma ICD10
C8415
C84.15 …… lymph nodes of inguinal region and lower limb
T cell Lymphoma ICD10
C8416
C84.16 …… intrapelvic lymph nodes
T cell Lymphoma ICD10
C8417
C84.17 …… spleen
T cell Lymphoma ICD10
C8418
C84.18 …… lymph nodes of multiple sites
T cell Lymphoma ICD10
C8419
C84.19 …… extranodal and solid organ sites
T cell Lymphoma ICD10
C846
C84.6 Anaplastic large cell lymphoma, ALK-positive
T cell Lymphoma ICD10
C8460
C84.60 …… unspecified site
T cell Lymphoma ICD10
C8461
C84.61 …… lymph nodes of head, face, and neck
T cell Lymphoma ICD10
C8462
C84.62 …… intrathoracic lymph nodes
T cell Lymphoma ICD10
C8463
C84.63 …… intra-abdominal lymph nodes
T cell Lymphoma ICD10
C8464
C84.64 …… lymph nodes of axilla and upper limb
T cell Lymphoma ICD10
C8465
C84.65 …… lymph nodes of inguinal region and lower limb
T cell Lymphoma ICD10
C8466
C84.66 …… intrapelvic lymph nodes
T cell Lymphoma ICD10
C8467
C84.67 …… spleen
T cell Lymphoma ICD10
C8468
C84.68 …… lymph nodes of multiple sites
T cell Lymphoma ICD10
C8469
C84.69 …… extranodal and solid organ sites
T cell Lymphoma ICD10
C847
C84.7 Anaplastic large cell lymphoma, ALK-negative
T cell Lymphoma ICD10
C8470
C84.70 …… unspecified site
T cell Lymphoma ICD10
C8471
C84.71 …… lymph nodes of head, face, and neck
T cell Lymphoma ICD10
C8472
C84.72 …… intrathoracic lymph nodes
T cell Lymphoma ICD10
C8473
C84.73 …… intra-abdominal lymph nodes
T cell Lymphoma ICD10
C8474
C84.74 …… lymph nodes of axilla and upper limb
T cell Lymphoma ICD10
C8475
C84.75 …… lymph nodes of inguinal region and lower limb
T cell Lymphoma ICD10
C8476
C84.76 …… intrapelvic lymph nodes
T cell Lymphoma ICD10
C8477
C84.77 …… spleen
T cell Lymphoma ICD10
C8478
C84.78 …… lymph nodes of multiple sites
T cell Lymphoma ICD10
C8479
C84.79 …… extranodal and solid organ sites
T cell Lymphoma ICD10
C847A
C84.7A …… breast
B Cell Lymphoma ICD10
Unspecified B-cell lymphoma, lymph nodes of multiple sites
B Cell Lymphoma ICD10
Unspecified B-cell lymphoma, extranodal and solid organ sites
B Cell Lymphoma ICD10
Unspecified B-cell lymphoma, lymph nodes of axilla and upper limb
B Cell Lymphoma ICD10
Unspecified B-cell lymphoma, lymph nodes of inguinal region and lower limb
B Cell Lymphoma ICD10
Unspecified B-cell lymphoma, intrapelvic lymph nodes
B Cell Lymphoma ICD10
Unspecified B-cell lymphoma, spleen
B Cell Lymphoma ICD10
Unspecified B-cell lymphoma, unspecified site
B Cell Lymphoma ICD10
Unspecified B-cell lymphoma, lymph nodes of head, face, and neck
B Cell Lymphoma ICD10
Unspecified B-cell lymphoma, intrathoracic lymph nodes
B Cell Lymphoma ICD10
Unspecified B-cell lymphoma, intra-abdominal lymph nodes
B Cell Lymphoma ICD10
Mediastinal (thymic) large B-cell lymphoma
B Cell Lymphoma ICD10
B Cell Lymphoma ICD10
Mediastinal (thymic) large B-cell lymphoma, lymph nodes of inguinal region and lower limb
B Cell Lymphoma ICD10
Mediastinal (thymic) large B-cell lymphoma, lymph nodes of axilla and upper limb
B Cell Lymphoma ICD10
Mediastinal (thymic) large B-cell lymphoma, spleen
B Cell Lymphoma ICD10
Mediastinal (thymic) large B-cell lymphoma, intrapelvic lymph nodes
B Cell Lymphoma ICD10
Mediastinal (thymic) large B-cell lymphoma, lymph nodes of head, face, and neck
B Cell Lymphoma ICD10
Mediastinal (thymic) large B-cell lymphoma, unspecified site
B Cell Lymphoma ICD10
Mediastinal (thymic) large B-cell lymphoma, intra-abdominal lymph nodes
B Cell Lymphoma ICD10
Mediastinal (thymic) large B-cell lymphoma, intrathoracic lymph nodes
B Cell Lymphoma ICD10
Mediastinal (thymic) large B-cell lymphoma, extranodal and solid organ sites
B Cell Lymphoma ICD10
Mediastinal (thymic) large B-cell lymphoma, lymph nodes of multiple sites
B Cell Lymphoma ICD10
B Cell Lymphoma ICD10
Diffuse large B-cell lymphoma, lymph nodes of multiple sites
B Cell Lymphoma ICD10
Diffuse large B-cell lymphoma, extranodal and solid organ sites
B Cell Lymphoma ICD10
Diffuse large B-cell lymphoma, intrathoracic lymph nodes
B Cell Lymphoma ICD10
Diffuse large B-cell lymphoma, intra-abdominal lymph nodes
B Cell Lymphoma ICD10
Diffuse large B-cell lymphoma, lymph nodes of axilla and upper limb
B Cell Lymphoma ICD10
Diffuse large B-cell lymphoma, lymph nodes of inguinal region and lower limb
B Cell Lymphoma ICD10
Diffuse large B-cell lymphoma, intrapelvic lymph nodes
B Cell Lymphoma ICD10
Diffuse large B-cell lymphoma, spleen
B Cell Lymphoma ICD10
Diffuse large B-cell lymphoma, unspecified site
B Cell Lymphoma ICD10
Diffuse large B-cell lymphoma, lymph nodes of head, face, and neck
References
1. ; Available from: https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/fda-investigating-serious-risk-t-cell-malignancy-following-bcma-directed-or-cd19-directed-autologous.
2. Kenneth, P.M., et al., Investigation of product-derived lymphoma following infusion of piggyBac-modified CD19 chimeric antigen receptor T cells. Blood, 2021.
3. Simon, J.H., et al., CAR+ T-Cell Lymphoma Post Ciltacabtagene Autoleucel Therapy for Relapsed Refractory Multiple Myeloma. Blood, 2023.
4. Kainat, S., et al., Second primary malignancies in patients with haematological cancers treated with lenalidomide: a systematic review and meta-analysis. The Lancet Haematology, 2022.
5. Brittany Knick, R., et al., Impact of second primary malignancy post–autologous transplantation on outcomes of multiple myeloma: a CIBMTR analysis. 2023.
6. Sharon Koorse, G., et al., Lenalidomide-Associated Secondary B-Lymphoblastic Leukemia/Lymphoma-A Unique Entity. American Journal of Clinical Pathology, 2020.
7. Jan, K., et al., Lenalidomide induces degradation of IKZF1 and IKZF3. OncoImmunology, 2014.
8. Philippe, K. and C. Susan, Role of Ikaros in T-cell acute lymphoblastic leukemia. World Journal of Biological Chemistry, 2011.




Interesting and timely reading. I would like to know how many of these so called T cell lymphoma are just clonal LGL T populations?