MIDAS touch is golden!
It is time to add quads including carfilzomib as the preferred proteasome inhibitor in the front-line therapy for multiple myeloma (MM)
Introduction
The MIDAS trial was just published and should be read carefully[1]. This should be the new standard for frontline therapy, transplant-eligible MM (TE MM). The trial was designed to test interventions based on minimal residual disease (MRD) results after induction therapy. In this initial report, the authors report rates of MRD negativity after six cycles. Importantly, in this trial, patients collected stem cells after three cycles - more extended therapy can make this more difficult. Most patients (99%) had at least one apheresis collection. The MIDAS trial included 791 patients who received isatuximab plus KRD. Carfilzomib was given weekly at 20/56 mg2, three times per month.
Efficacy
The best overall response rate (ORR) was 95%, and 92% achieved a VGPR or better. MRD rates in the intent-to-treat (ITT) population were 63% at 10⁻⁵ and 47% at 10⁻⁶. As the “per protocol” design, the MRD-negativity rates were 66% at 10⁻⁵ and 50% at 10⁻⁶. No significant difference was observed between the rates of MRD negativity for patients with high-risk disease (vs those with standard risk). However, patients with 17p del or t(11;14) had lower rates of MRD (48 and 40%, respectively). This seems common for t(11;14) and discussed elsewhere.
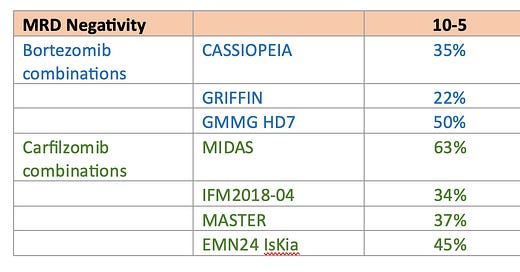
MRD-negativity (at a threshold of 10-5) after induction was 63%, compared to 35%, 22%, and 50% in the CASSIOPEIA, GRIFFIN, and GMMG HD7 trials, respectively, which used quadruplet combinations with bortezomib (four 28-day cycles in CASSIOPEIA, four 21-day cycles in GRIFFIN, and three 42-day cycles in GMMG HD7). As compared to IFM2018-04, MASTER, and IsKia, the MRD rates were also superior
SAFETY
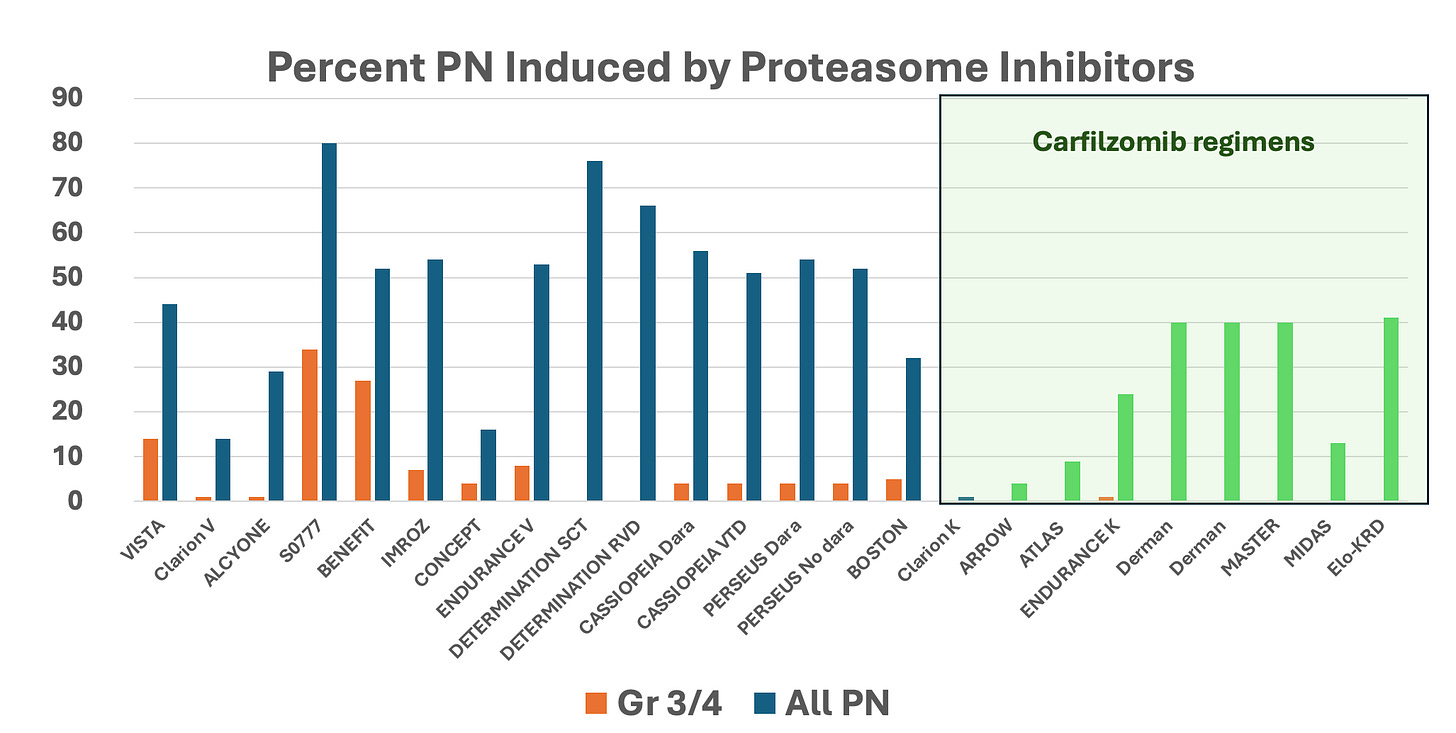
Treatment-related mortality was low at less than 1%. There were only two cardiac deaths (0.02%), and both were in patients with known coronary disease. Peripheral neuropathy was minimal and reported in only 13% of patients. The grades were low, with 10% being grade 1 and 3% being grade 2. These results are much better than those reported with bortezomib-based regimens (see graph below).
Clinical trials rate of peripheral neuropathy
Rates of peripheral neuropathy with bortezomib and carfilzomib (green box) containing regimens. The blue color represents all grades of peripheral neuropathy in bortezomib clinical trials while the green one represents the rate of peripheral neuropathy (any grade) in carfilzomib clinical trials. Orange bars represent grade 3 or 4 peripheral neuropathy regardless of proteasome inhibitor used.
What can we learn from this?
Six is the new 4! Why do we administer four cycles of induction therapy? Only because it is a legacy of times when we used VAD. Back then, it was hard to tolerate more than four cycles, each with 12 days of high-dose dexamethasone. Plus, you had the cumulative effect of vincristine and adriamycin.
It is key to remember to collect stem cells after three cycles. A previous French study had shown a failure of up to 20% of stem cell collection if this was attempted after six cycles of therapy.
Data continues to point toward carfilzomib having better efficacy than bortezomib. It is a recurrent theme to see more profound responses and higher rates of MRD negativity with induction regimens that contain carfilzomib. In addition, I would argue that carfilzomib is safer overall than bortezomib. Serious cardiac adverse events were rare in the MIDAS trial. Importantly, patients were selected to avoid cardiovascular complications. Skeptics will argue that endurance failed to show the superiority of carfilzomib over bortezomib because of the ENDURANCE Phase 3 trial. This was not an equivalency trial and had some design aspects that make us careful with its generalization (e.g., exclusion of high-risk patients).
But even if I were to concede equivalent efficacy the safety profile is much more favorable for carfilzomib. In regimens containing carfilzomib, neuropathy is milder and less common than in regimens containing bortezomib. The BENEFIT trial employed weekly and subcutaneous bortezomib but still had a high rate of neuropathy - ~52%, with 27% of patients having it grade 2 or higher. Additionally, it is essential to note that bortezomib-induced neuropathy is enduring.
It is time for NCCN guidelines to include KRD in combination with anti-CD38 antibodies (isatuximab or daratumumab) regimens to be added as preferred options for frontline MM therapy. I often need to do “peer-to-peer” appeals to secure patient insurance coverage. The MIDAS study shows better and safer results.
Why does the MM community continue to have a preference for bortezomib over carfilzomib? I presume some leaders may believe that weekly and subcutaneous bortezomib is quite safe, but BENEFIT clinical trials have shown this to be not true. Some might worry that a higher cost of carfilzomib may harm patients or health systems. If I were to be diagnosed with MM, please keep bortezomib away from me!
[1] https://ashpublications.org/blood/article/doi/10.1182/blood.2024026230/535235/Isatuximab-Carfilzomib-Lenalidomide-and





I love everything about this post. Direct, understandable, and the concluding discussion doesn't equivocate. Plus it underscores my growing belief that patient education shouldn't be all-consuming. A very few times a year -- and more often, every few years -- of getting information like this to discuss with your healthcare teams is much more helpful than treating disease information like it's a headline news feed that has to be consumed constantly. Take the time to digest information like this, it will be much more productive and more likely to remain sane.
Thank you. After my diagnosis 6+ years ago with the kappa-only version of mm, plus GI amyloid, I received CyBorD and did very well but developed severe neuropathy. That led to dara and 5 mg Rev, which kept me at very low numbers; but after seeing MRD numbers not be as low as they could be, I substituted venetoclax for Rev and it has been amazing. Still no relapse, ever, or illness--or sct. I found it interesting that the 11;14 people in the study did not do as well...would be interested in that other discussion you mentioned. Totally agree with staying away from bortezomib!!!